KYSA-6 phase 3 clinical trial is enrolling!
Investigating a new therapy to help people living with generalized Myasthenia Gravis (MG).
What is the KYSA-6 phase 3 clinical trial?
60 participants with generalized MG to receive a therapy called KYV-101
The trial will compare KYV-101, an investigational CAR T-cell therapy, to standard of care treatmenta
Multiple leading medical centers
Eligible participants will have tried previous treatments, but their disease is still not well controlled
KYSA-6 is a phase 2/3 trial. Phase 3 is an expansion of KYSA-6 and is taking place in multiple leading medical centers in the US and around the world. The phase 2 portion of the KYSA-6 study is fully enrolled. Please contact us for additional information and to find out which site might be nearest to you.
Who can take part in the KYSA-6 phase 3 clinical trial?
You may be able to participate in the KYSA-6 phase 3 trial if:
You are 18 to 75 years old.
You have been diagnosed with generalized MG according to specific criteria.
You have tried previous treatments, but your disease is still not well controlled.
You are positive for acetylcholine receptor (AChR) or muscle-specific kinase (MuSK) autoantibodies.
This list does not include all the trial participation criteria and additional criteria may apply.
What will happen in the KYSA-6 phase 3 clinical trial?
If you are selected to participate, one of the doctors running the trial (or someone on their behalf) will talk with you about what you can expect as a participant in the trial so that you understand and are comfortable with what it means to take part. This is called informed consent.
If you are able to enroll in the trial, these are the key steps you will be involved in:
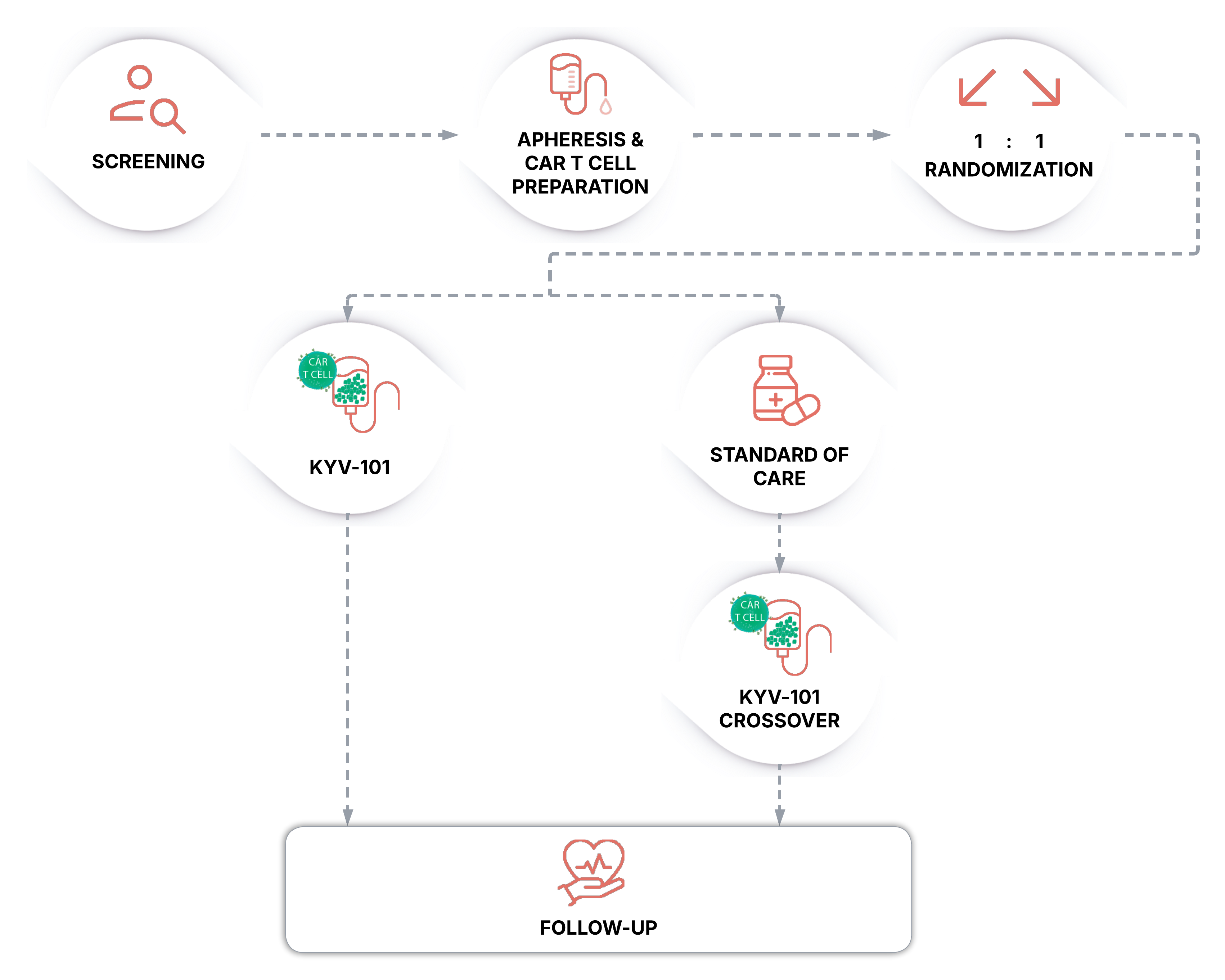
CAR T-cell therapies can be associated with cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS), which may be potentially serious or life-threatening but generally resolve within the first month after treatment.
- Symptoms of CRS include fever, nausea, feeling tired (fatigue), and body aches and can progress in severity and may include low blood pressure, shock, and potentially organ failure.
- Symptoms of ICANS include fatigue, uncontrolled movements (tremors), impairment in thinking, loss of speech, muscle weakness, or more severe symptoms such as seizures and swelling in the brain.
People receiving this treatment are always in the care of a doctor, who monitors them closely for these side effects and can treat their symptoms to help prevent their worsening.
If you are interested in taking part in the trial, your doctor can refer you to a trial site or you may reach out directly via this site to learn more and be referred. If you are selected for screening, the trial doctor will assess you to confirm your eligibility for trial participation. This may include performing additional tests. The trial doctor will also explain the detailed criteria for participating. This trial has multiple sites in the United States and internationally. To participate in this clinical trial, you will need to travel if you do not live near a trial site.
Participant Accommodations: We aim to make every participant's KYSA-6 clinical trial experience as easy as possible. Accommodations for trial participation may be available and may include travel and caregiver support.
Click here for additional information and details on our clinical trial participation criteria.Clinical Trial Resources
KYSA-6 Flyer for Patients and Caregivers

KYSA-6 Flyer for Healthcare Provider Discussion

MG Patient Support Groups
Patient support groups can offer additional educational resources and support for people managing MG and those who care for them.b

Conquer MG
Conquer MG is an organization that supports, informs, advocates, and funds research for MG, with the goal to directly impact patients and their families impacted by MG.

Myasthenia Gravis Association
The Myasthenia Gravis Association (MGA) is an organization dedicated to supporting and empowering patients, families, and communities impacted by MG.

Myasthenia Gravis Association of Western Pennsylvania
The Myasthenia Gravis Association of Western PA is an organization with the mission to address the medical, social, and emotional needs of all persons affected by MG and to disseminate educational information to persons with MG, their families, the medical community, and the general public.

Myasthenia Gravis Foundation of America
Myasthenia Gravis Foundation of America (MGFA) is the largest, leading patient advocacy organization solely dedicated to finding better treatments and a cure for the disease while improving the lives of those living with MG. The MGFA fosters collaboration across medical and research communities and raises funds that support innovative MG research.

Myasthenia Gravis Foundation of Michigan
The Myasthenia Gravis Foundation of Michigan (MGMI) is a Michigan-based organization that provides patient support and advocacy, fosters connections within the MG community, and advances education for MG.

Myasthenia Gravis Holistic Society
MG Holistic Society is an inclusive wellness-centric community that offers a fresh perspective to the myasthenia gravis (MG) disease management paradigm. It is our mission to empower MG patients and their families to recognize that MG is a part of life, but life is more than MG. Learn more at mgholisticsociety.org
MG can significantly affect a person's life. Those with MG can be their own best advocates by learning about MG and its treatments, tracking their symptoms and medications, staying in touch with their care team, and connecting with advocacy groups.
Learn more about MG →aStandard of care may consist of traditional agents for MG (eg, prednisone, azathioprine, mycophenolate, methotrexate, chronic IVIG/PLEX) or complement pathway inhibitors (eg, eculizumab, ravulizumab). Neonatal Fc receptor(FcRn) inhibitors within 4 weeks or rituximab (or any other anti-CD20 or CD19 moncolonal antibody) within 12 weeks prior to screening not allowed.
bNote that these links are provided for informational purposes only and its content is not necessarily supported or endorsed by Kyverna Therapeutics.
Contact us about KYSA-6
For more information, please contact Kyverna by completing the form.

